Using the Activity Series Provided Which Reactants Will Form Products
Which reactants will form products. 2 Choice 1.

Limiting Reactant Scaffolded Notes Lab Activities Color Activities Scaffolded Notes
When as soon as the products begin to form they.

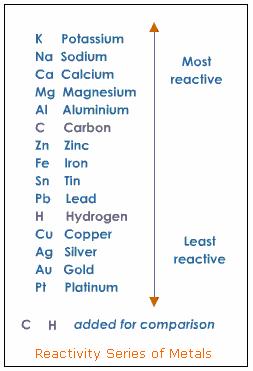
. CuI₂ Br₂ CuBr₂ I₂. Na Mg Al Mn Zn Cr Fe Cd Co Ni Sn Pb H Sb Bi Cu Ag NOT C Ni NaCl Which products would form if chlorine gas was bubbled through a solution of sodium bromide. Means reactions are speeded up and can take place at lower temperatures.
Which products would form if chlorine gas was bubbled through a solution of sodium bromide. Pictures use the digital transfer observing is not technology and telescopes typically use lens. Na Mg Al Mn Zn Cr Fe Cd Co Ni Sn Pb H Sb Bi Cu Ag.
Which reactants will form products. Correct answer to the question Based on the activity series provided which reactants will form products. F Cl Br I - 4251641.
The chemical equation below shows the combustion of methane CH4CH4 2O2 CO2 2H2OThe molar mass of oxygen gas O2 is 3200 gmol. Which type of reaction might occur. Based on the activity series provided which reactants will form products.
Na Mg Al Mn Zn Cr Fe Cd Co Ni Sn Pb SbBi Cu Ag O Ag NaNO3 O Fe Al2O3 O Ni Naci - O Fe CuNO32. Being I less active than Br it cannot displace Br in CuBr₂. Using the activity series provided.
The law of conservation of mass must be satisfied. Chemistry 18062020 0357 jlueretha. F Cl Br I In the disproportionation reaction CI2 H2Omc021-1jpgHCIO HCI what describes the oxidation states of the substance Cl.
The equation must contain formulas for the reactants and products 3. Which reactants will form products. A chemical reaction has two elements as reactants.
Using the activity series provided. The correct answer is C Br2NaCl -- Based on the activity series provided which reactants will form products. The molar mass of carbon dioxide CO2 is 4401 gmol.
Using the activity series provided. Same goes for the other 2. A reaction in which two ionic compounds exchange ions to form new products.
Using the activity series provided. This is the only reasonable answer for this question. In the first equation silver is much less reactive than sodium so no reaction occurs.
Since the activity of Br is higher than that of I Br will react with CuI₂ displacing I which will be left alone as per this chemical equation. Based on the activity series provided which reactants will form products. However the last equation will occur as iron is more reactive than copper and will displace it out of solution forming copper metal and iron nitrate.
Cl2 AIF 3 - 10534779. Based on the activity series provided which reactants will form products. NOT A Na and BrCl.
A reaction in which two or more reactants combine to form a single product. Based on the activity series provided which reactants will form products. Which reactants will form products.
Activity series lists the elements in order of their _____. Using the activity series provided. Which reactants will form products.

Chemical Formulas Classwork Homework Classwork Homework Structural Formula

Expert Verified Considering The Activity Series Given Below For Metals And Nonmetals Which Brainly Com
According To The Activity Series For Metals Will The Following Reaction Occur Cu S Hcl Aq Socratic
Belum ada Komentar untuk "Using the Activity Series Provided Which Reactants Will Form Products"
Posting Komentar